When to Use Nitrocarburizing / Nitriding Article written by Dr. Edward Rolinski
Nitriding has an old history of development and applications. It has been used for more than 100 years to improve wear and corrosion resistance as well as fatigue strength in many engineering components made of steels or cast irons [1-8]. This process was modified and enhanced very quickly by introducing carbon-bearing gases to the atmosphere and causing alloying of the upper portion of the hardened layer, resulting in even better properties of the layer. In this way, nitrocarburizing was introduced. The precipitation of nitrides and carbonitrides as well as the nitrogen supersaturation of the ferrite, respectively, cause an increase in surface hardness and generation of compressive residual stresses [3].
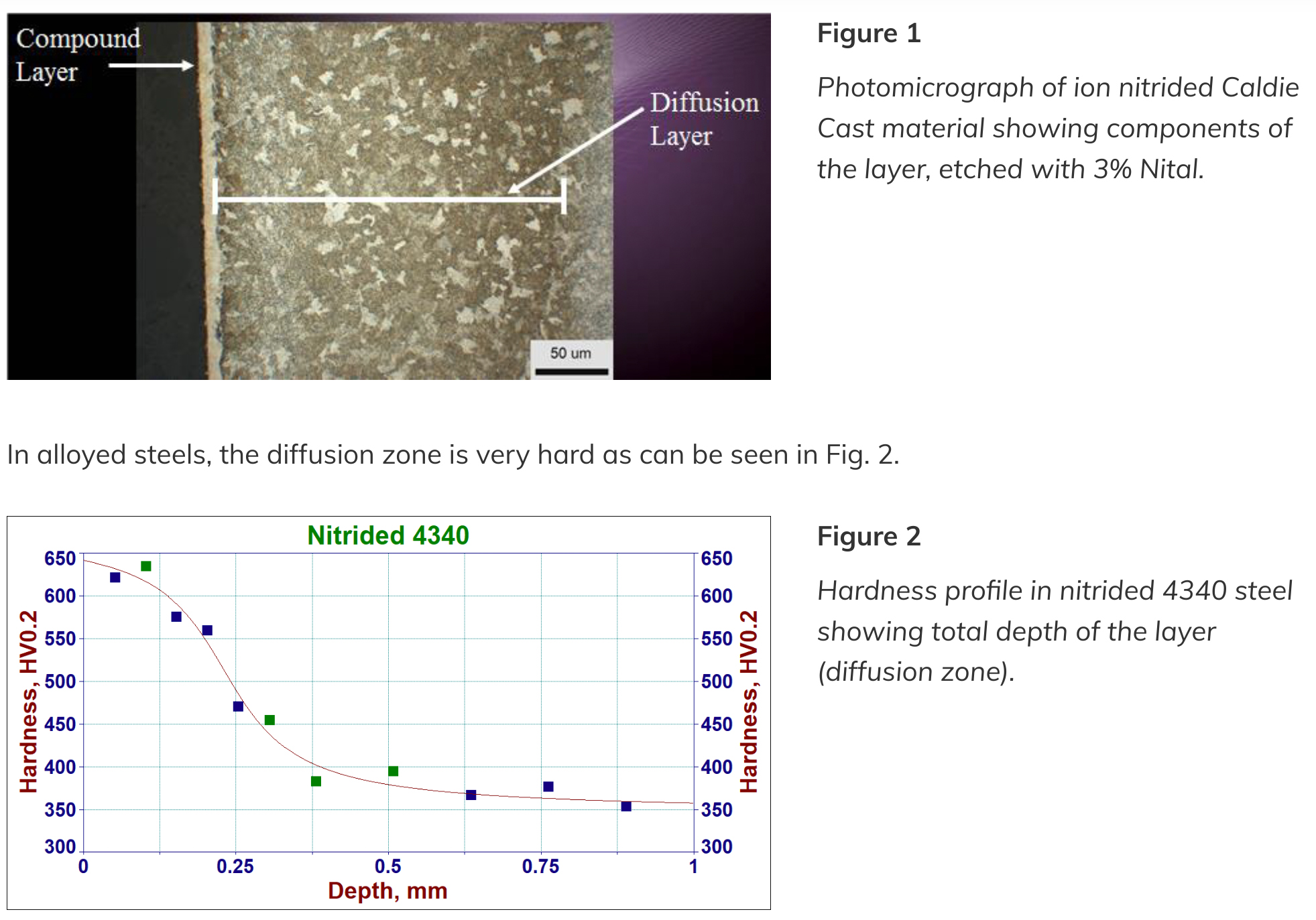
Depending on the specific application, the process can be adjusted to produce a layer with the proper structure required for it. The nitrided layer has essentially two sublayers; the compound zone (CZ) at the surface also called white layer (WL) and below, a layer formed by nitride precipitations called the diffusion layer (zone), see Fig. 1.
Gas Nitriding
Nitriding is done typically within temperature range 400-590°C (752-1094°F), which is below Ac1 temperature of the iron-nitrogen system and because of that, it is called ferritic nitriding or just nitriding [1-6]. If processing temperatures exceed the upper temperature range, nitrogen austenite is formed resulting in the presence of an additional sub-layer which its structure depends on the cooling rate [3, 4].
Nitriding Compound Zone (CZ)
The compound zone structure depends on the process parameters and the chemical potential of nitrogen used. The Lehrer diagram describes those parameters and the resultant nitrogen content in iron. Utilizing the Lehrer diagram allows for prediction and control of the amount of nitrogen introduced into the surface of steel [1-6]. At low nitriding potential, CZ is thin or could be completely eliminated, see Fig. 3.
As can be seen above, when nitriding potential is high, thickness of the CZ can be significant and it may contain the Epsilon nitride with the higher nitrogen content. Presence of this nitride accelerates growth rate of the CZ, see Fig. 3 and 4.
Properties of the CZ depend on its structure and the amount of nitrogen in it. Also, as mentioned before, thickness and properties of CZ can be modified by introducing carbon atoms in it.
Nitrocarburizing
Nitriding modified/doped with carbon is for increasing CZ thickness to the range 10-40 µm (0.0004-0.0016”) and formation of Fe3(C, N)1+x phase and the process is called Nitrocarburizing.
The reactivity of the process gases used for gas nitriding and nitrocarburizing treatments is characterized by means of the potentials of nitrogen, carbon, and oxygen, which are defined as nitriding potential, carbon potential, and oxidation potential [3].
Gas Nitrocarburizing
This process is called nitrocarburizing and also FNC (Ferritic Nitrocarburizing) [2]. FNC is an extremally important method of surface hardening for the components made of low-alloy, low-carbon steels although it is quite often also used for other steels when a high hardness as well as excellent corrosion resistance are required. Optimization of the structure for superior corrosion resistance is achieved when both nitriding and carburizing potentials of the nitrocarburizing atmosphere are controlled and when percentages of nitrogen and carbon introduced into Epsilon Layer (Fe2CN) is about [C]/ [N + C] = 0.02–0.2 [3, 6].
Gas nitrocarburizing shall be carried out in an automated mode, maintaining set parameters of temperature and nitriding with carburizing potentials as recommended by ASM Standard [1]. The process is controlled and maintained automatically at each stage. Recommended values of nitriding and carburizing potentials for temperatures are also listed there.
Nitriding Potential and Carburizing Potentials are defined in the following way:
The nitriding potential is a measure of the nitriding capability of the nitriding atmosphere, which determines the surface nitrogen concentration in iron at a given temperature, and is described by the following equation:
Carburizing Potential
The carburizing potential is a measure of the carburizing capability of the carburizing atmosphere, which determines the surface carbon concentration in pure iron at a given temperature, and is described by either of the two following equations [1]:
The differences between the hardness profiles of nitride layers of unalloyed and alloyed steels are well known. Hardness of diffusion zone in unalloyed steel is very low. Therefore, hardness of the treated component depends on presence of the sufficiently thick compound zone. In situations like that nitrocarburizing is the proper option. It provides sufficient wear protection of the surface. Its corrosion resistance is also very high, much better than resistance of the compound zone produced by nitriding and chromium plating [3, 6].
It should be noted that additional corrosion resistance can be achieved when there is a magnetite oxide layer produced during post-oxidizing step on top of the compound zone [6].
On the other hand, if a good bending and rolling fatigue resistance of the high alloy steels, such as M-50 or 4340 is needed, the best properties are achieved when compound zone is absent or minimized. In those situations, a good diffusion with a high hardness needs to be produced. In those situations, nitriding is used.
In contrast, today, in the case of bath FNC, a deliberate change of the effective chemical potentials of nitrogen and carbon, is still not possible. Therefore, in these processes, the control of the structure of the nitrided case is empirical [3].
Ion Nitriding & Nitrocarburizing
Ion/plasma nitriding and nitrocarburizing is another option for treating ferrous alloys components [7, 8]. This method is excellent when treating very precise parts requiring local protection from the treatment, especially those made of stainless steels or sintered metals. The method is characteristic of a “low-nitriding potential” which allows to limit easily thickness of the compound zone even without very sophisticated control of the process. In many applications, the epsilon type CZ can be produced by doping plasma with methane or other hydrocarbon. Thickness of the compound zone can be limited to not more than 10 µm without loosing all the properties and benefits of such a layer. Those results cannot be obtained so easily when the other methods are used.
Who is Doctor Glow?
Dr. Edward Rolinski, aka Doctor Glow, has been studying the plasma/ion nitriding phenomenon since the 1970s and is arguably one of the most knowledgeable people in North America when it comes to nitriding.
The doctor has written countless articles and whitepapers in industry publications and manuals. Some of his most noteworthy contributions include the chapter on “Controlling Plasma Nitriding” in ASTM International (2017) as well as “Nitriding of Titanium Alloys” in the ASM Handbook (2016).
Did you like this article? Click here to subscribe to The Monty.
View our recent magazines and podcasts by clicking the following link. https://themonty.com/magazine/