Tool Steel and its Heat Treatment Part I By; David Pye
This presentation is the first of three parts and is focused on the heat treatment of tool steels. The complete presentation will address the categorization of tool steels, followed by heat treatment practice and concluding with troubleshooting, which will address some failures due to;
- Tooling design
- Possible machining practice
- Material selection
- Design of Experiment
- Quenching practice
- Tempering
Tool Steel Categorization
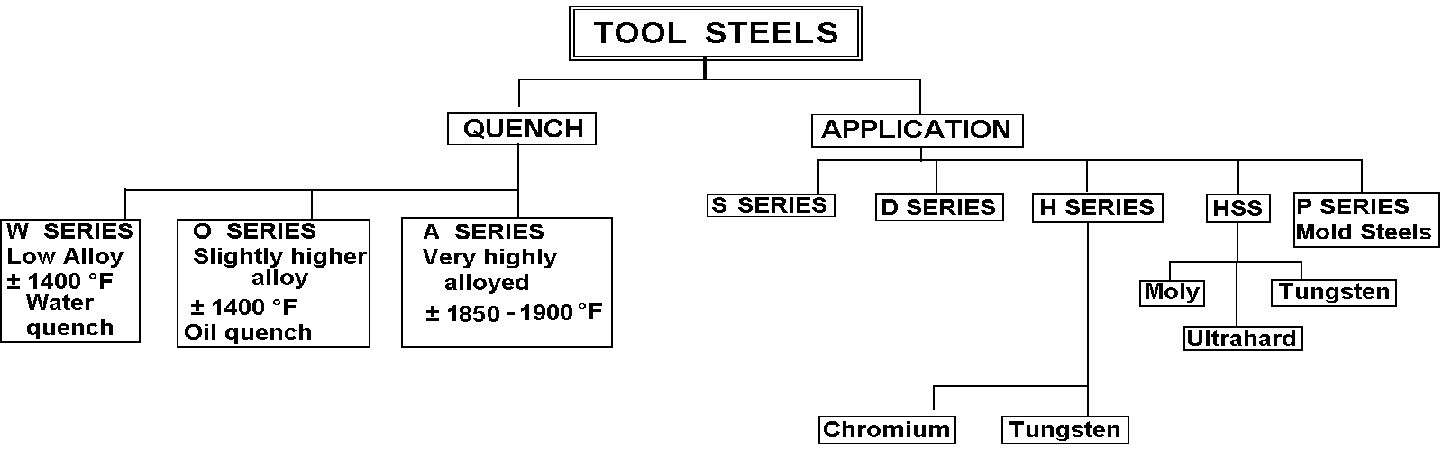
The illustration below is a suggestion as to how tool steels are generally categorized into one of two categories. Those categories are Quenching and application,
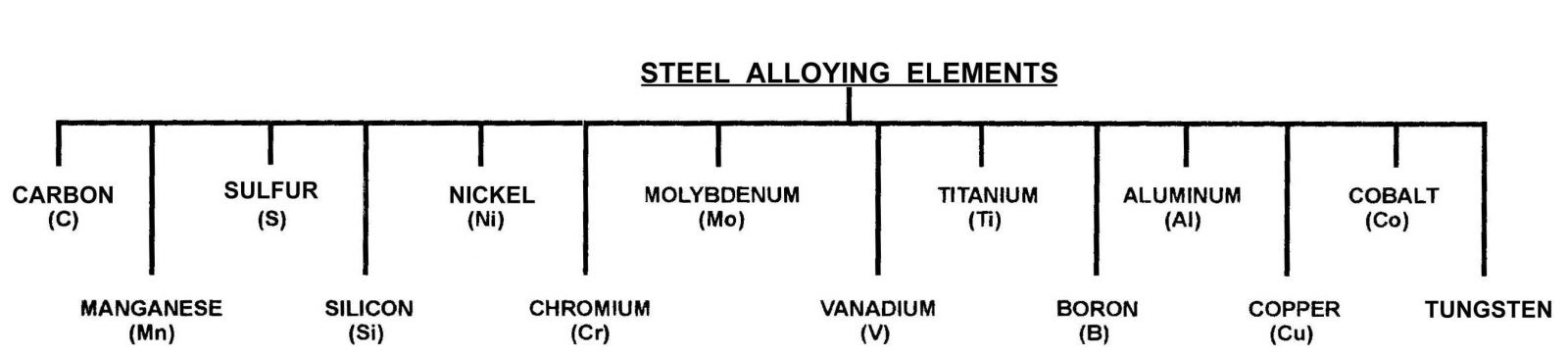
Above is shown in illustration of alloying elements which can be present in a tool steel to address different operational characteristics. (Please bear in mind, that the alloying elements will affect the selection of hardening and tempering temperatures.
- W1 to W5 = Low temperature hardening with water quench.
- O1 to O7 = Low temperature hardening with oil quench.
- A2 to A9 = Higher temperature high alloy, air hardening
- D2 to D7 = High carbon, high chromium cold work tool steels with a high austenitizing temperature and complex quenching
- S1 to S7 = Shock resisting tool steels
- H (Chromium) H10 to H19 = Hot work die steel with high operating temperature. Also, high hardening temperature with complex quenching.
- H (Tungsten) H21 to H26 = Hot work tool steels with higher operating temperatures and high hardening temperatures plus complex quenching
- H (Molybdenum) up to H42 = As above for H21 to H 26.
- HSS (TUNGSTEN) = T1-T15Complex steels designed for high speed cutting.
- HSS (Molybdenum) = M1 – M36. As above
- HSS (Ultra Hard) = M41 – M47As above
- L2 – L6 = Low alloy special purpose tool steels.
- Mold steels = P2 – P21 Mold steels, usually low carbon and used often for cold hobbing of the impression on coining dies, then carburized or sometimes nitrided.
Influence of alloying elements in Tool Steels
Carbon is what makes iron turn into steel. The amount of carbon that is added to steel will determine its maximum hardness and mechanical properties. It can be said of carbon, that carbon is the magic ingredient that is added to iron to create hardness. The amount of carbon present in the steel will determine the steels maximum hardness value.
MANGANESE (Mn)
Manganese is present in all steels, including alloy steels as well as Tool Steels. There is no dividing line between carbon steels and manganese alloy steels. Generally, Manganese is present in amounts above 0.6%.
It is used to reduce oxide formations (deoxidizer) and it will form with sulfur to reduce embrittlement in the steel.
It also assists as a low-cost hardener in some of the lower alloyed Tool Steels. If it is present with Chromium and Molybdenum it will help to resist deformation on air hardening. Usually found in the A series Tool Steels.
CARBON (C)
Carbon is the universal hardener is all steels. It can be found in amounts from 0.01% up to 2.3%. It does not require a significant amount of carbon to affect the hardness of the steel. This element will interact with the elements listed below to form carbides. Once the carbon content approaches or even surpasses amounts greater than 1%, it will usually combine with other elements such as:
- Chromium
- Molybdenum
- Vanadium
- Tungsten
- Cobalt
- Titanium
SULFUR. Sulfur is considered to be an impurity and at the primary steelmaking it is deliberately reduced. However, there are instances when it is necessary to add sulfur in controlled amounts to improve the steel’s machinability.
SILICON. This material is used primarily as a deoxidizer during the steel making process. However, in large amounts it will begin to affect the steel’s ductility. However, in the high alloy heat resisting steels, it will assist in the resistance to oxidation at high temperatures.
CHROMIUM
Chromium is found in a wide range of tool steels and in varying amounts. Chromium is one of the elements that has a tendency to form carbides with Carbon in the steel curing the heat-treatment procedure. Chromium will assist in:
- Deep hardening
- Slight improvements in corrosion resistance
- Wear resistance
- It can be a disadvantage if the tool steel is held too long at austenitizing temperature by causing grain growth.
TUNGSTEN
Tungsten will raise the hardening temperature (Austenitizing temperature). It forms very stable carbides with carbon. It will inhibit grain growth at elevated temperatures. It is found extensively in High Speed Steels which usually will form the excess carbides within the matrix of martensite. It’s primary function is to give high red heat hardness in both High Speed Steels and Hot Work Steels.
Vanadium
Vanadium has two primary functions in tool steels: As a grain refiner, and a stabilizer of carbides at high temperatures. It will have a stabilizing effect on martensite. This function makes it difficult to temper. The cycle times on tempering tend to be longer and require multiple tempering
MOLYBDENUM
Molybdenum will also form the complex carbides with carbides. It will improve the deep hardening characteristics of the steel. It is found in tool steels such as:
- Hot Work
- High Speed Steel
- Usually found at concentrations of around 4% plus.
- It also makes the steel resistant to tempering and also assists in the “secondary hardening” characteristics of the steel.
COBALT
This element is not usually seen in large quantities and is more usually found in the super alloy special High-Speed Steels. It will however tend to reduce the steel’s hardenability (not hardness) It will tend to improve a High-Speed Steel’s cutting ability. Because it will reduce the hardenability it will be necessary to increase the carbon content. It is usually found in steels such as T15 and M33.
