Nitriding, Process Methods and Metallurgy
From international heat treat consultant David Pye [email protected] we have an article about Nitriding.
The process Nitriding is a diffusion process and not a deposition process. It is a diffusion procedure that is reliant on a nitrogen source, and a low thermal process temperature (which, by looking at the Iron Carbon Equilibrium diagram is in the ferrite/cementite region of that diagram) just as the process of carburizing and carbo-nitriding processes are.
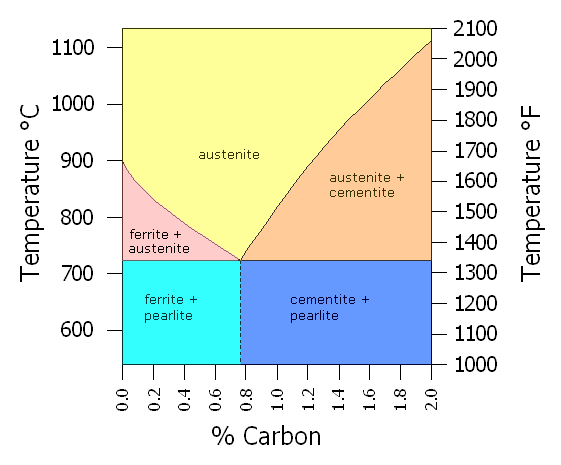
However, there are significant differences in the two surface hardening process techniques;
- A lower process temperature (determined for steel by selecting a process temperature below the steels prior tempering temperature by some 50°F to say 70°F, and of course making considerations for the possible potential for distortion).
- Surface metallurgy and mechanical properties (different surface phases and diffusion zone metallurgy)
Machlet of Elizabeth, New Jersey was granted a process patent granted in 1908. The German metallurgist (Dr. Adolph Fry of Krupp Steel) was granted a European process patent in 1923. This led Krupp Steel to develop and market selected steels which were then known as Special Nitriding Steels or better known as the Nitralloy steels by Thomas Firth and John Brown Steels in the United Kingdom.
The process principle is based on the simple premise of the limits of solubility of nitrogen in iron. In other words, nitrogen will diffuse in the iron or steel very easily with the application of heat and will interact with the some of the alloying elements. (This also includes Iron)
The chemistry of the process is extremely simple and uncomplicated. The source of nitrogen is ammonia for gaseous nitriding. The surface reaction and the decomposition are as follows:
- 2NH3 + heat 2N + 3H2 (Equation 1)
- 2 N +3H₂ → N₂ +3H₂ (Equation 2)
- Kn = pNH ₃/pH₂ 3/2 (Equation 3)
The initial decomposition is to separate the nitrogen from the hydrogen of the ammonia (nitrogen source). For a fraction of a second, the nitrogen is atomic, which will react with the steel being treated which then diffuses into the steel surface. The hydrogen acts as a dilutant gas and also a reducing gas to assist in reducing surface oxide contaminants. The steel surface acts as the catalyst to assist in the gas decomposition.
The ratio of nitrogen to hydrogen in the ammonia decomposes to a ratio of 1 part nitrogen to 3 parts of hydrogen. This is a very important observation to note when defining what type of formed immediate surface metallurgy is required for the application of the part being nitride.
From the decomposition of the gas, atomic nitrogen will react the steel surface and begin to diffuse into the steel. The nitride forming elements that will react with the atomic nitrogen and form stable nitrides are as follows:
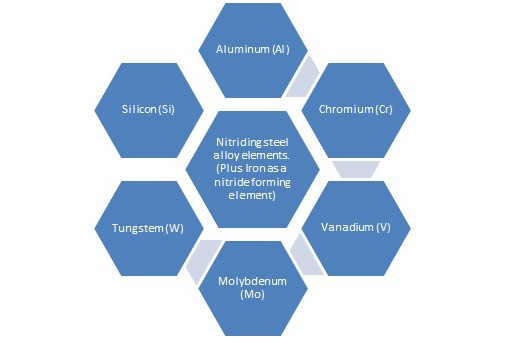
It should not be forgotten that Iron is also considered as a nitride former. However the resulting nitrides do not exhibit high hardness values, but assist in the improvement of the steels resistance to corrosion.
Nitriding methods.
At least 4 different, yet basic different methods of applying the nitride process to steel and are:
- Gaseous nitriding (using ammonia as the nitrogen source)
Ammonia gas molecule
- Salt bath nitriding (using a cyanide based salt such as KCN and NaCN) (Potassium Cyanide and Sodium cyanide)
- Plasma nitriding process techniques can also be known also as Glow discharge nitriding, or Plasma nitriding Continuous DC nitriding, Pulsed Plasma nitriding.
The decomposition of the ammonia to release both nitrogen and hydrogen diffusion is very similar with each of the above methods except with the Plasma nitriding. Time, temperature and material chemistry will also influence the;
- Surface metallurgy
- Surface hardness
- Core hardness
- Diffusion zone enrichment
- Nitride networks
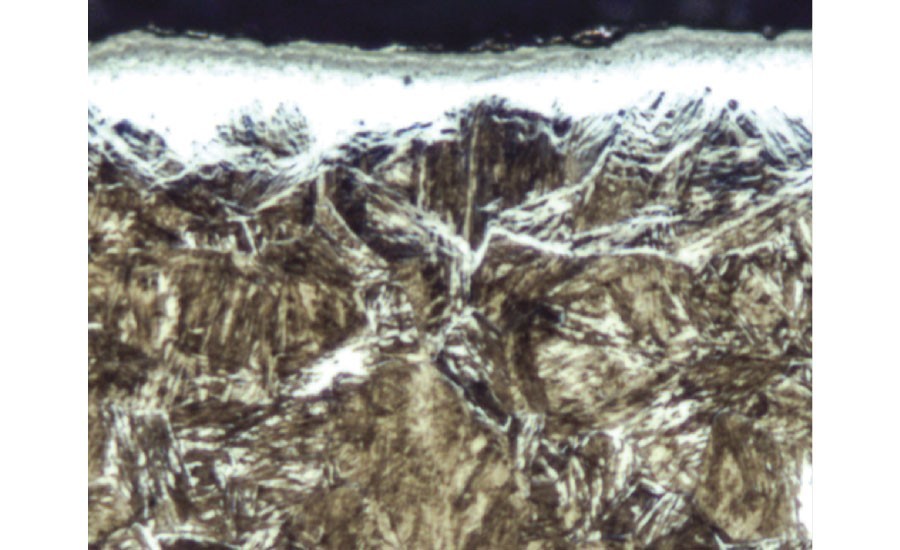
Severe Nitride Networks
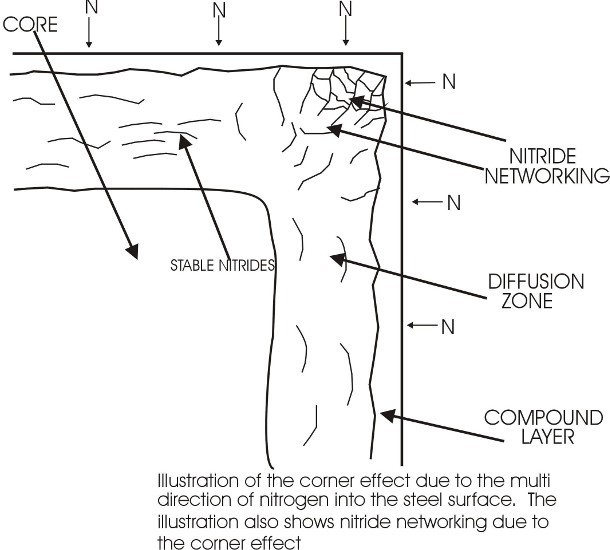
Metallurgy
The final metallurgy (As seen in the Illustration below and by the gaseous process methods or salt bath methods) will be formed as seen in the lower illustration) comprise of the following results in the order of observation (microscopically)
- Compound layer ( also known as the white layer), because the immediate surface etches out white with a cross sectional examination of the formed case) The compound layer comprises of two phases known as Gamma Prime (ỳ) and Epsilon nitride (έ) The compound zone formation can be controlled by adjustment of the process gas flows(or salt bath chemistry)
- Diffusion zone. This is where the nitrogen has reacted with the alloying elements to form stable nitrides with the appropriate alloying elements.
- The transition zone. (this is the area from the diffusion zone to the core material)
- This is the original core metallurgy of the steel being nitrided.
The exception to the above will be nitriding by plasma techniques and nitriding by dilution techniques.
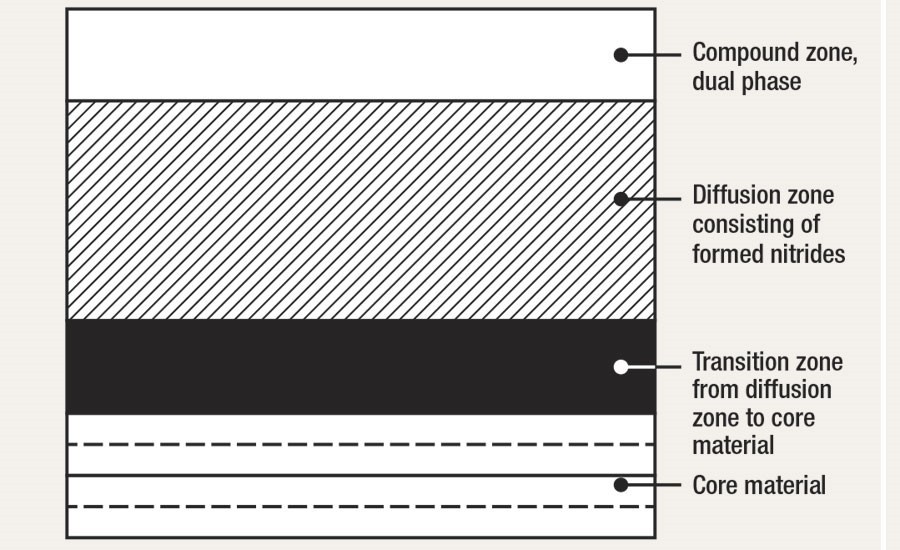
General nitride surface metallurgy of the formed case
Plasma
What do we understand as “plasma”? There are many forms of natural plasma,such as;
- Lightening
- Aurora Borealis (Northern Lights!
A simple generated fluorescent light is a plasma light source with can be colored by various internal gases.

Plasma Ball

Ball Studs under Pulsed Plasma Assisted Nitriding Conditions
The thermal process requirement can be accomplished by Continuous DC generated Plasma, or be a combination of variable pulsed plasma, in conjunction with an electrical external heat source
Plasma nitriding has the distinct advantage of being able to control the ratio of nitrogen to hydrogen in order to control the resulting surface metallurgy of the nitriding process. The formed compound zone can be constructed of:
- Dual phase (ỳ and έ)
- Single (Mono) phase (ỳ or έ)
- No compound layer
It can be said that “fixed process chemistry = fixed resulting surface metallurgy, but variable process chemistry = variable surface metallurgy”
This can simply be accomplished, by control and adjustments of the ratios of molecular nitrogen to hydrogen.
Steels for Nitriding
Any steel will nitride, simply because of the presence of iron. However they will not produce the same hardness values because of the steel chemistry’s. The iron will assist in the surface corrosion resistance by the formation of Iron Nitrides.
Below is a general group of steels that will nitride. The list is by no means complete:
- Cast Irons. Cast iron grades will nitride without any significant difficulty. The problem then arises because of the cast iron density and The ability of the nitriding to nitride cast iron has been known for many years and is not new. A new use has been developed for cast irons, which is the nitriding of cast iron forming dies for the surface hardening of large auto bodies (such as the tractor in tractor/trailer. This has been pioneered by European and USA die manufacturers with commercial heattreaters.
- Alloy steels. Most alloy steels will nitride. However care needs to be taken when considering the choice of steel for nitriding and in particular with the carbon content. It is not generally necessary to have a high carbon percentage in the steel to give high core hardness in order to support the formed case. A carbon content of approximately 0.45% maximum is considered acceptable. Once again, please be aware that the carbon content of the steel will affect the ratio of gamma prime to epsilon phases in the compound layer.
- Tool steels. The typical tool steels for nitriding will be the Hot Work series of tool steels. The High Speed Steels will nitride very satisfactorily, as will the Air Hardening tool steels. There are some applications where the D series are nitrided, but care should be exercised when selecting the D series for nitriding.
- Stainless steels. All of the stainless steels will nitride. This is because of the ability of chromium to form high surface hardness values. However some will nitride easier than others. The martensitic stainless steels are perhaps the easiest to nitride. All of the other stainless steels require some form of surface de-passivation to remove the chrome oxide layer on the immediate surface of the stainless steels. Once the chrome oxide surface layer, then the stainless steel has lost its corrosion resistance.
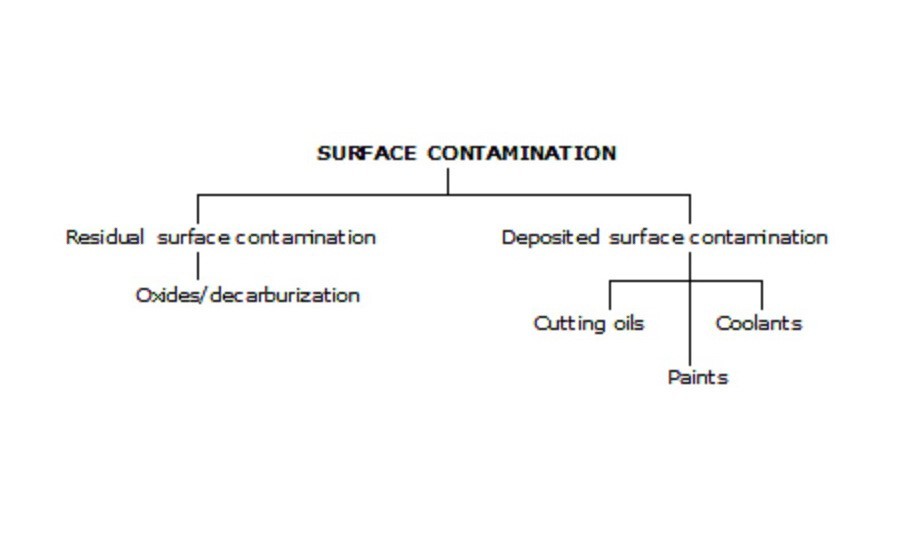
A general note for accomplishing a good and sound nitrided structure.
It is a mandatory requirement that the surface is free from surface contamination such as is seen below
Illustration of possible surface contaminations
Sincerely David,
Pye Metallurgical International Consulting
www.heat-treatment-metallurgy.com
Hampton Va 23669
Tel;757 968 1007
Representing Ionitech of Sofia Bulgaria
