An Understanding of the Annealing and Normalizing Process’s By David Pye Part 1
A Simple Review of the process of Annealing.
We know that the purpose of annealing is to make the steel soft and malleable. We know that the process of normalizing will provide a uniform prior grain structure and flow. We know that the process of stress relieving will reduce induced stresses in the steel as a direct result of cold working and machining of the steel.
Why is carbon added to the steel?
Carbon is added to iron to transform the iron into steel, because that is what steel is. Simply an alloy of iron plus carbon. However, the more carbon that is added to the steel, the worse the surface finish will become. The strengthening effect of carbon in steel is the fact that the carbon is in solid solution in the steel. The grain size and type of phase formation is an important consideration to the machining characteristic of the steel as well as it ability to respond at the final heat treatment. The grain size grain orientation will also influence the steel’s ability to resist (not eliminate) cracking at the final heat treatment. Once the carbon percentage exceeds 2.3%, then the steel is becoming cast iron. Cast iron can exist with either graphite nodules in the form of round nodules, or long stringers of graphite. Cast iron can also exist as white or gray cast iron.
The cast irons are simply iron with an excess or super saturated solution of carbon in iron. Cast Irons do not usually have any carbide forming elements present in solution. Because cast irons have excellent compressive strength, they have been traditionally used in machine tool applications. It is interesting to note now, that in present day machine tools other materials are being used for compressive strength.
The following illustration shows the effects of carbon in a plain carbon steel. It further shows the importance and effects the quench medium and the transformation of austenite to martensite. Please remember that this is for a plain carbon steel, not an alloy steel or tool steel. The top line displays the hardness results based on a good transformation to martensite, and the bottom line shows the effects of only 50% transformation from austenite to martensite.
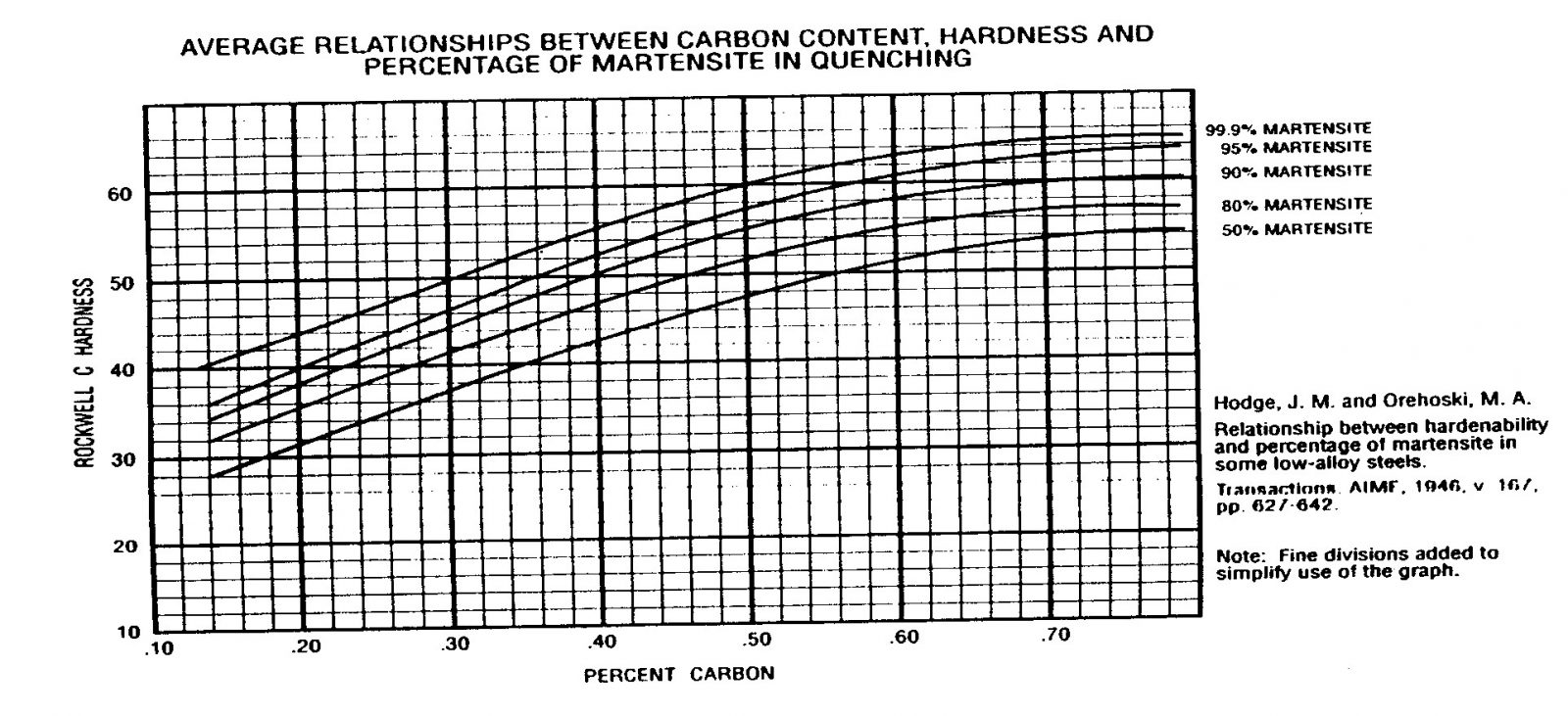
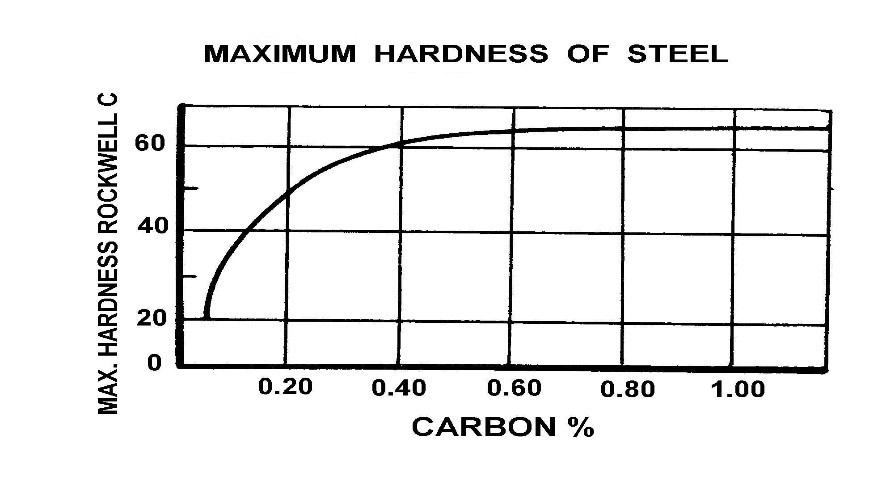
The previous illustration (shown above indicates) the approximate maximum hardness achievable despite the increase in carbon % above approximately 0.65The following illustration once again shows, the principle alloying elements that are typically added to the steel ladle when the steel is being made.
There are other elements which are used as trace elements and are not shown here. Each of the alloying elements creates different properties and reactions within the steel when the steel is austenitized, quenched and tempered.
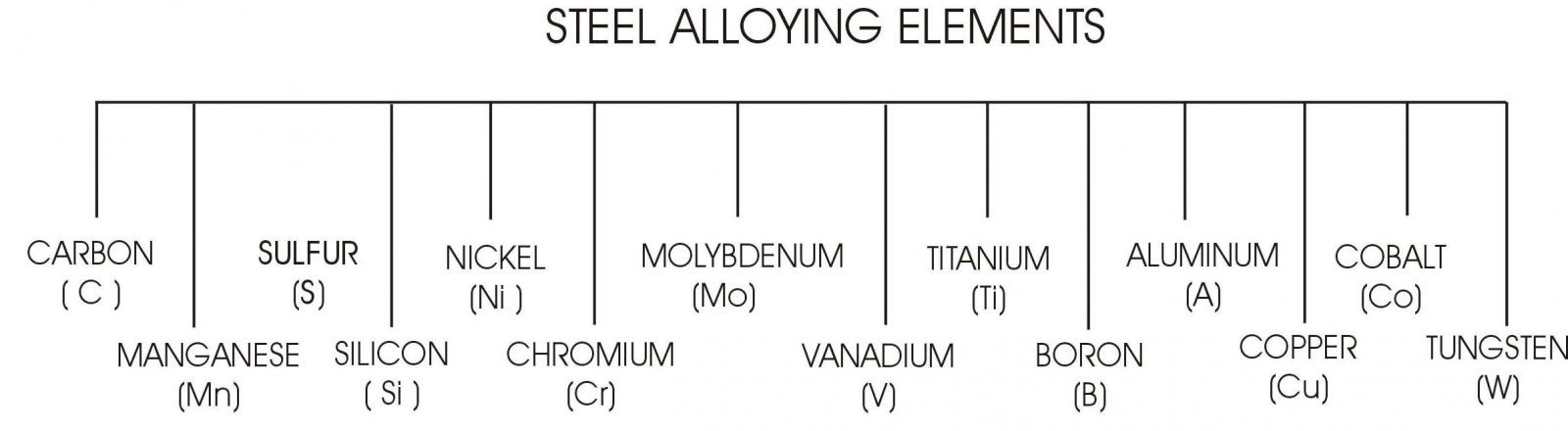
Manganese
Manganese is used as a deoxidizer and desulfurizer. It is usually present in amounts from 0.30% up to a maximum of 2.0%. However, there is a steel called Hadfield’s Manganese steel with 12.00% manganese and 1.00% carbon. The steel is soft (austenitic) at room temperature but will rapidly work harden making it very useful for high abrasive wear. The influence on the hardness properties of steel by the introduction of suitable alloying elements.
Silicon
Silicon is present in most steels as one of the principle deoxidizers at the steel melt operation. It is usually kept to a maximum content of 0.40% maximum. If that value is exceeded, then a reduction in ductility will be seen. Silicon will also assist in hardenability if combined with manganese or molybdenum.
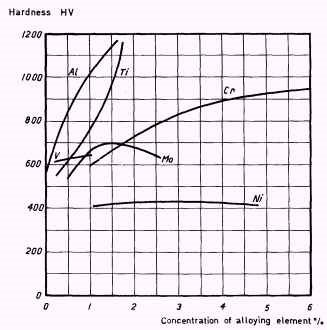
A graphical interpretation of the change in the mechanical properties due to various elemental additions to an alloy steel.
The illustration below is of a plain carbon steel exhibiting a coarse grain structure.
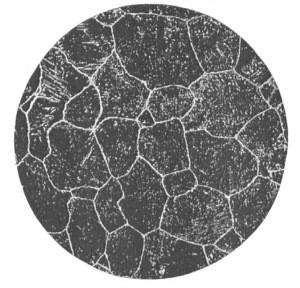
Comparison of coarse grain steel in relation to fine grain steel.
- Greater distortion factor than with fine grain steels.
- Deeper depth of hardening than with fine grain steels.
- Lower ductility factor than a fine grain steel of the same analysis and hardness.
Sulfur is present in the first instance as a trace impurity. Sulfur is also added as an element to assist with free machining capabilities.
- High sulfur will also cause segregation at the grain boundaries in significant amounts.
- If the steel is a bearing steel, then sulfur will improve the fatigue life.
- In the free machining steels, the sulfur is anywhere betwe0.08% up to 0.32% ca
- If there are high manganese contents and high sulfur contents, the sulfur can react to form insoluble manganese sulfide stringers which are insoluble.
Phosphorous
- Phosphorous is also seen as an impurity in high concentrations, of greater than 0.04%
- Phosphorous has the ability to reduce both ductility and impact toughness.
- High phosphorous contents can also cause temper embrittlement in the alloy steels, and it can also help and assist with hardenability.
Aluminum
- Aluminum is a strong deoxidizer at the steel making procedure, and also a grain refiner.
- Aluminum is also a strong nitride former and will react to for hard nitrides.
- Aluminum can also assist in the reduction of surface oxide formation. Do not be under the impression that it will eliminate surface oxidation, it will only reduce it.
- The higher contents of aluminum are generally added to the heat resistant alloys to reduce the oxidation problem.
- Aluminum can also be added to improve the corrosion resistance of low carbon corrosion resistant steels.
Chromium
- Chromium is a carbide former and is used in tool steels as well as alloy steels to form hard carbides with carbon.
- Chrome carbides assist in the resistance of abrasion due to their very hard nature.
- The chrome carbides will retain hardness values at high operating temperatures.
- Chromium will improve the corrosion resistance of a steel as well as assist in the reduction of surface oxidation.
Molybdenum
- Molybdenum is an exceptionally strong carbide former in the presence once again of carbon.
- Molybdenum has the ability to improve the secondary hardness characteristic of some of the highly alloyed tool steels.
- Molybdenum will assist very strongly as a grain refiner.
- Molybdenum will improve both hardenability and fatigue strength.
- Molybdenum also has the ability to improve the corrosion resistance of a steel, although not as well as chromium.
- It is also used to reduce the creep strength of low alloy steel that operate at elevated temperatures.
- Molybdenum is also used in some stainless steels to help reduce pitting corrosion.
Boron
- Boron is generally seen in steel in very small amounts of between 0.0005% up to 0.0035%.
- It will improve the depth of hardening very dramatically of all of the other alloying elements in solution in the steel.
- Large amounts of Boron make it very brittle. Its only benefits are seen with the lower concentrations.
- Boron is also a useful element in assisting a steels weldability.
Nickel
- Nickel is a non-carbide former and will do nothing in the steel to assist with the formation of both carbides and nitrides.
- Nickel will raise the hardenability of certain steels.
- Nickel will also assist in improving fracture toughness and fatigue resistance.
- However, in a carburizing steel, high nickel contents tend to promote retained austenite.
Tungsten
- Tungsten is among the carbide formers and will readily for strong carbides. As the tungsten content increases, the carbide formation will increase.
- High percentages of tungsten will assist in the secondary hardness phenomenon in certain tool steels.
- The presence of tungsten will enable the steel (high speed steels and hot works) to retain their high hardness values at red heat.
- Tungsten will also minimize the risk of grain growth at elevated temperature. Therefore, tungsten is a grain refiner.
Cobalt
- Cobalt is not a carbide former, and on its own it will reduce the hardenability of steel. However, if cobalt is present with chromium, the steels hardenability will improve dependent on the percentages of both elements.
- Cobalt will raise the Martensite Start line on the Tim Temperature Transformation diagram.
- Cobalt will also inhibit grain growth.
- Cobalt will further improve the temper and high temperature strength.
- The use of cobalt is usually restricted to the high alloy tool steels, such as HSS Hot Works.
Vanadium
- Vanadium is an excellent carbide former and will strongly improve the steels hardenability.
- Vanadium is also a strong nitride former and will for strong nitrides.
- Vanadium is also a grain refiner and will improve the tensile strength and toughness.
- Vanadium is used in the micro-alloyed steels as a strong carbide and nitride dispersion.
- Vanadium will also assist in tempering and it will raise the steels ability to cut and it will raise the steels ability to cut and retain its hardness at red heat.
- Vanadium will also help to retain the edge of a cutting implement because of the strong carbides.
- It will also assist in the ease of welding of heat treatable steels.
Lead
- Lead has been the element of choice to add to steel for free machining properties.
- Lead has been used in a range of 0.20% up to 0.50%
- Lead will begin to evaporate from the surface of free machining steels at elevated process temperatures such as carburizing. It will leave a slight surface porosity.
- The use of lead as a free machining element is declining almost to the point of extinction, because of it being hazardous to health.
Nitrogen
- Nitrogen is usually present in solution with the steel.
- However, it can be added as a solid solution by the decomposition of (for example) ammonia.
- Nitrogen is also an austenite stabilizer. In other words, during the process of carbo-nitriding, Nitrogen will suppress the Upper Critical Lines of the Iron Carbon Equilibrium diagram, thus reducing the austenitizing temperature. (Reducing the distortion).
Niobium and Tantalum
- Niobium and Tantalum are strong carbide formers, but are not present in large percentage amounts.
- Both elements are also strong grain refiners and will increase the yield strength of the steel.
- Niobium is used predominantly in micro alloyed steels to create an increase in the steels yield strength.
Copper
- Copper is not a usual alloying element in steels. Copper will increase the steels hardenability
- The presence of copper will improve the corrosion resistance of steels if present in appreciable amounts. If above 1% in stainless steels, it will improve the resistance to acidic corrosion.
Zirconium
- Zirconium is added to HSLA steels to improve the steels resistance to nonmetallic inclusion formation such as occurs with manganese sulfide.
- Zirconium is also a very strong carbide former and will react with readily with carbon in the steel to form zirconium carbides dispersed through the matrix of the steel.
We will continue with part 2 in the next issue. Sincerely, David
