An Introduction to Carburizing (Part 2)
We are focusing initially in this particular presentation (Part 2) on the pack carburising. The last leg of the first presentation shows five methods of carburising.
- Pack Carburizing process.
- Molten Salt Bath process.
- Gaseous Atmosphere Carburizing process.
- Low Pressure Carburizing (Vacuum)
- The Plasma Assisted Carburising process.
Pack Carburizing compounds are generally made up in the following manner with activators.
- Wood Charcoal = 90%, plus Barium Carbonate = 10%
- Wood Charcoal = 90%, plus Sodium Carbonate = 10%
- Wood Charcoal = 75%, plus Barium Carbonate = 15%,
- These are simple ‘home made’ methods of making carburising granulate.
Carburizing Surface Reactions.
The carburizing container (that is filled with the carburising granulate) will always contain air. When heat is applied, the air will interact with the carburising granulate and release both nitrogen and oxygen under thermal conditions.
At the selected process temperature and the approach up to the process temperature, the thermal condition will cause the oxygen to react with the carbon to form carbon monoxide.
Once again due to the thermal conditions the carbon monoxide will react with the steel surface to be carburized and release nascent/atomic carbon plus carbon dioxide. It is the nascent carbon that is diffused into the surface of the steel component shown in the following reaction sequence.
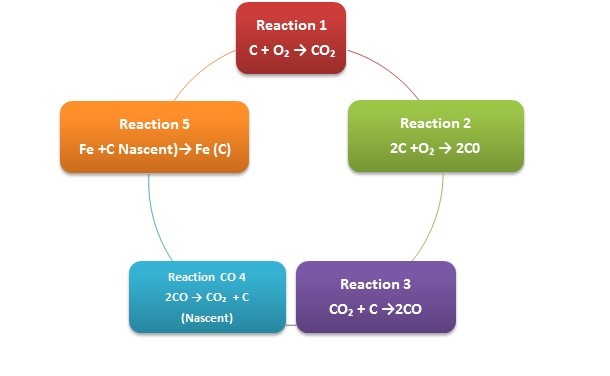
Surface Reactions during the Pack Carburizing Process.
Replenishment of used Activated Charcoal.
The charcoal will be the medium that supplies the carbon for diffusion into the surface of the steel and the activator will assist in the reduction of carbon dioxide to the surface of the steel being carburized.
After the completion of each charge or load, it will be necessary to replenish the used charcoal with an approximate addition of 10 to 12% by volume of new activated charcoal.
A simple loose fitting steel lid should be placed on top of the loaded container and simply sealed with fireclay or mud to contain the process gases developed within the container during its complete cycle at temperature.
When preparing the process container for the charcoal granulate and the steel to be carburised, it will be necessary to ensure that there is approximately 50 mm (2 inches) of charcoal combined with the activator underneath the steel to be process and over the top of the steel component being processed.
Internal Potential Contamination by Grain Boundary Oxidation of the New Charge Being Processed.
When pack carburizing it must always be remembered that the presence of oxygen within the process retort is being derived from the decomposition of air that is initially present in the process box. This will give rise to the formation of intergranular oxidation, (or grain boundary oxidation, GBO). The depth of the oxidation will depend on the process cycle time in relation to the case depth required. Obviously, the deeper the case depth, the deeper the GBO.

An example of Inter Granular Oxidation
Influence of Time on the Process of Carburizing
The time factor is a complex formula and based upon the diffusion of carbon into the surface of the steel. The rate of elemental diffusion into the steel surface is not dependent upon the element that is being decomposed. (Such as carbon or nitrogen)
The principal formula is based upon Fick’s first and second laws of diffusion. As previously stated these two formulas are very complex. Because of the formulation is quite a complex formula, Harris derived a much simpler formula for the time of diffusion for a given case depth which was based on a plain carbon steel (without alloying elements).
Fick’s Simple law of Diffusion (Modified by Harris)
The rate of diffusion will follow the law of diffusion as defined by Harris;
Case depth = √? x ?
t = time
f = factor (derived from the process temperature)
The formula is based on simple plain carbon steel and does not account for alloy carburizing steels, but can be used simply as a guide. It does not take into account the effects of alloy elements such as chromium, nickel and any other alloying element that may have been added.
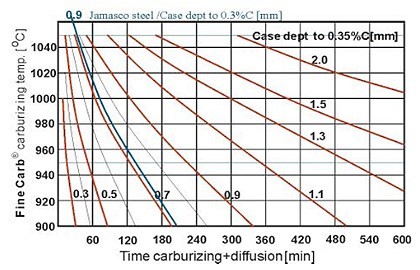
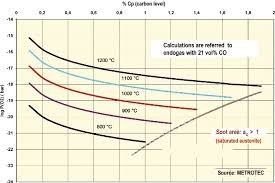
The depth of formed case must be tested for hardness after quenching. (Note: after quenching for the hardening procedure of the newly formed case).
Depending on the selected load on the hardness test machine will determine if carburizing has taken place or if the indenter has pierced the formed case and is giving an average reading between case and core. Therefore it is strongly recommended that a cross sectional traverse hardness plot be given to accurately establish the total depth of newly formed case.
The depth of carbon penetration is (as previously described is a function of time and temperature in order to diffuse the carbon into the steel surface.
The depth of hardness penetration will be dependent on the carbon content of the carburized layer. Provided that only martensite is formed in the case when quenched, the depth of case hardening would be equivalent to a depth of carbon penetration down to 0.4 % carbon.
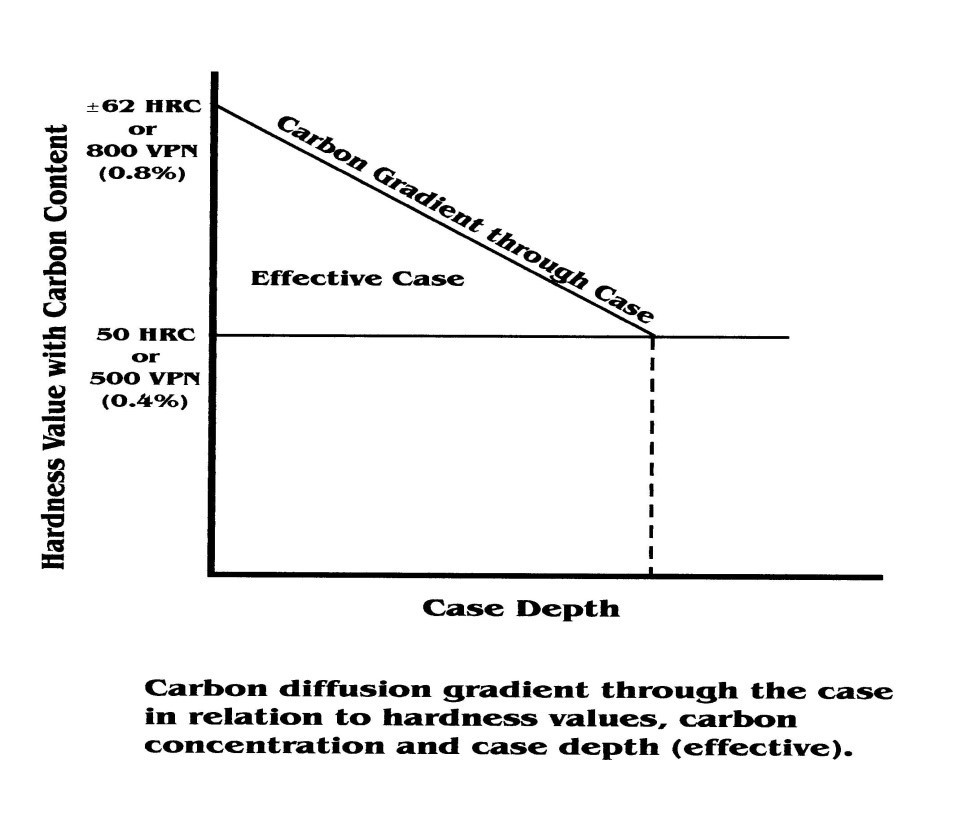
The core hardness will be dependent on the austenitizing temperature chosen to harden the formed case. If the selected austenitizing temperature goes too high, then the core hardness will be correspondingly high.
If the selected austenitizing temperature is too low, then there is a likelihood of a softer core hardness and the potential for incomplete transformation to occur on the formed case.
The core hardness will also be dependent on the chemistry of the steel in question.
The purpose of the core hardening treatment is to give support to the fresh martensite formed case.
If the hardness of the core is too low and the load that could be applied to the surface of the formed case, then there is a strong likelihood that the formed case will collapse due to lack of core support.
When carburizing by the pack carburizing method then the steel component (if necessary) can be selectively carburized and quenched. Generally, for pack carburizing, the method of stop off will be either copper plating or copper painting. There are other reputable stop-off agents that can perform as good as the copper plate stop off method.
When pack carburising, the most serious difficulty is establishing the time at which the components to be carburised and the process container have reached the selected carburising temperature.
The general selected process temperature for pack carburising is between 890°C up to 920°C.
The process furnace heating chamber will reach the set point carburising temperature before the contents of the process container reach the selected temperature.
Remember, when you observe the process container in the furnace process chamber, you are only seeing the outside of that process container. You are not observing the interior of the container.
Based on the Harris formula, the following approximate values can be used to approximate the process cycle time at a process temperature of 1650°F up to 1700°F.
The values given below are based upon the Harris Formulae and a plain carbon steel, shown as follows:
Case Depth = 0,016” T at 1550° F
Case Depth = 0,018” T at 1600° F
Case Depth = 0,021” T at 1650° F
Case Depth = 0,025” T at 1700° F
Case Depth = 0,030” T at 1750° F
Case Depth = 0,036” T at 1800° F
Case Depth = 0,043” T at 1850° F
Case Depth = 0,051” T at 1900° F
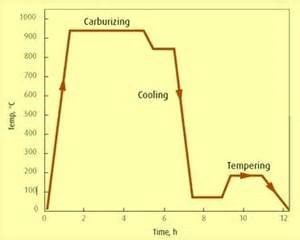
A typical Carburizing cycle with suggested temperature‘s
To be continued with Atmosphere Carburizing

